| Table of Contents |
Imagine that five years from now your notebook is being read by someone who knows just about as much physics as you do, but isn't familiar with the particular experiment you're doing. Will he be able to understand what you've done and what conclusions you drew? Will he be able to repeat your work? To compare his results meaningfully with yours? Even, perhaps, to spot where you went wrong? If you can't say "yes" to all these questions, then you could be keeping a better lab notebook.
You aren't required to write formal lab reports on these experiments. Instead, your laboratory notebook itself is turned in for grading and comments after each lab. EVERYTHING you've done, both in the lab period and after, goes into this notebook, IN ITS FINAL FORM, WHEN IT HAPPENS. Your lab notebook is not a place to do some formal final report, but a live logbook that you keep all through the course of the experiment starting with your preparation before the lab and continuing through the setup, taking data, analysis, and final conclusions.
Being aware of what you're doing while in the laboratory, and keeping a careful and thorough record of everything that happens there, are much more important than getting the "right" result. The cardinal virtues of a good laboratory notebook are completeness and clarity. These are not the same things as length and beauty!
"Completeness" just means that everything that happens appears in the notebook. All your work is done in ink, directly into the notebook. When you make mistakes, you line them out neatly and add a note, explaining why. Nothing is ever erased or blotched out your mistakes and corrections are part of your lab record too. Never tear pages out of your notebook. If extra pages must be included, such as special graph paper, they should be fastened in permanently (taped or glued) at the appropriate point.
All conditions that might be relevant to your experiment should be recorded. If you make a note of the ambient temperature, and then don't need it, nothing is lost; but if it turns out you do need it, and didn't record it, you're out of luck. Your account should show all your false starts, misunderstandings, burned-out meters, etc. as well as the time it finally worked.
. . . and "clarity" is what keeps this kind of completeness from turning your logbook into a terrible mess of blotched pages and non sequiturs. If you think ahead about what you're going to be doing next, and lay out your data in tabular form, clearly labeled and dimensioned, and constantly write down what's going on at each stage, you can produce an account that is thorough and complete and yet legible. Your hypothetical reader will be able to tell not only what happened to you, but where everything fits into what you were trying to accomplish.


You must do your work in a bound notebook, not loose-leaf or spiral. When you first get it, number its pages, all the way through, on both sides. (Leave the first page or two blank for an index.) This will make it much easier to keep things organized. Whenever you draw a graph, you reference the page on which the data was taken; if a calculation or argument must be broken off before it is finished, you can refer to the page on which it's picked up again. This kind of cross- referencing takes no effort, and it allows you to keep a log of things as they happen, interweaving data, calculations, and discussion as needed.
There is no one "right" way to keep a lab notebook, and I won't try to lay out a specific format for you. (Your lab instructor will probably have specific rules of his own that he wants you to abide by, however.) In most cases, your experience with a particular experiment consists of four phases, and your writeup should reflect the same general structure: introductory matter, procedure in lab, analysis of data, and conclusions.
Those parts of your writeup that can be thought through and carried out as soon as you come into the laboratory, or sometimes even in advance. Your account should begin with the title of the experiment, the date on which the lab work was done, and the names of the students who performed it. You'll always want some brief discussion of what it is you're trying to measure, of what law or hypothesis it is you're trying to test. This is the place for a brief summary of the relevant physical theory. Don't forget your (supposed) reader knows (at least) as much physics as you do; a lengthy "theory" section is almost never called for here.
Cutting out the Introduction or Procedure sections from the experiment writeup and pasting it in your notebook is bad practice. You'll want to keep your lab manual unmutilated, and they usually contain a lot more than needs to go in your notebook.
Another good way to handle the introduction is to leave one third or one half a page blank at the very beginning of your account and, when you're done, write an abstract of your account. (If you do this, label it clearly as an "Abstract"; an abstract isn't the same thing as an introduction!) An abstract is a very short (100 words or less) yet complete summary of the experiment: what you did, what you got, and what conclusions you drew. Published scientific papers are always headed by an abstract.
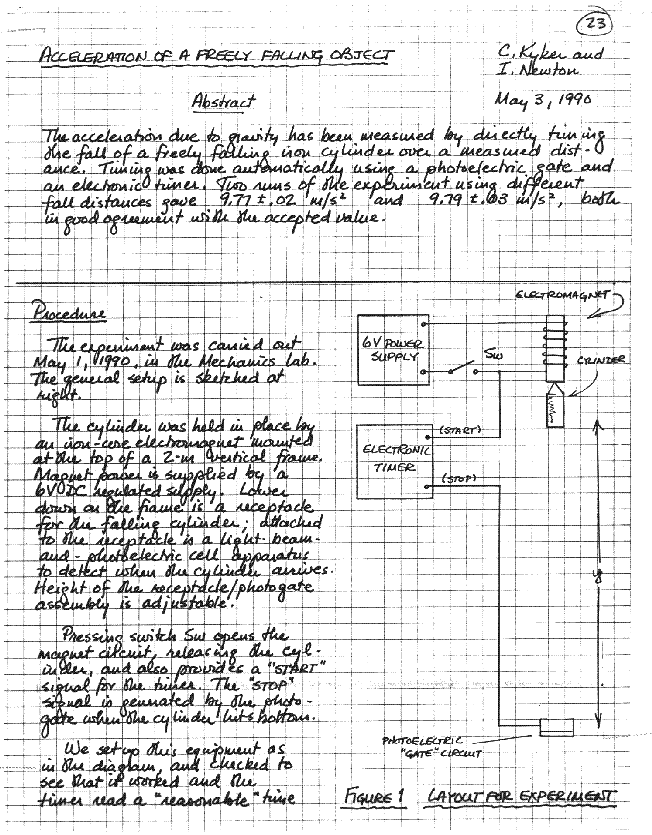
An account of everything you do, and everything that happens to you, in the course of carrying out the experiment. A description of the experimental apparatus should appear here. Each item of equipment should be listed, with identification numbers for specific instruments wherever available. Almost every experiment requires one or more diagrams to make the setup clear, and those should appear here too.

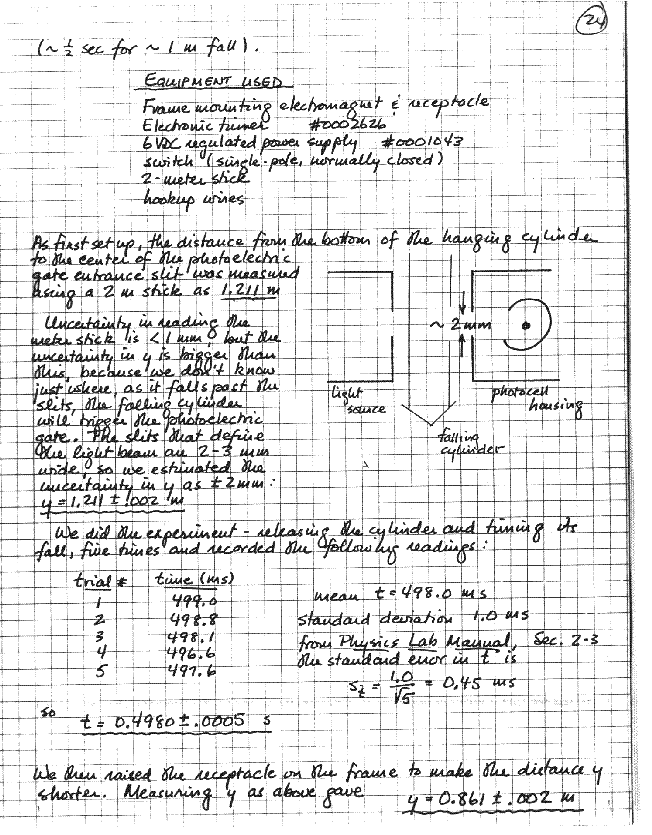
Taking data is not just a matter of writing some numbers down. False starts, miswired circuits, dead oscillators, burned-out meters, spurious resonances, and so forth are important too. What you're doing, and why, and how well, must be written down right there with the numbers, in enough detail so that there can be no ambiguity, later, about what the numbers mean. As an example, one of the PH 245 labs deals with standing waves on a stretched string. A parameter that must be known is the linear density (mass per unit length) of the string. Here's the entire entry from one student's record:
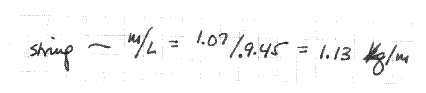
In effect, he's done nothing but list his result. "m" and "L" aren't defined; the conditions of measurement aren't recorded; quantities are stated without units; no estimate of uncertainty has been made; and so on. After our hero got his lab book back (covered with red ink), he rewrote the account; the new version is shown below.

Everything is defined, conditions of measurement are made clear, an estimate of uncertainty is given for each quantity. (I'll go into how the uncertainty in m/L was found in the next chapter.) The difference between these two samples is the difference between unsatisfactory and thoroughly satisfactory lab work.
There is a reason for all of this. Imagine that, a couple of years from now, you are trying to figure out why this guy's results in this experiment were a couple percent off. It occurs to you that maybe he forgot to measure the linear density of the string under tension but no, he mentions that. Could anything about his instruments have been fishy? Well, a meter stick is pretty much idiot- proof; what about the analytical balance? . . .and you go and check balance PH000123, and find a damaged knife-edge in the suspension that makes it read consistently 15 mg high. The discrepancy in this student's results has enabled you to avoid lots of future bad results but only because he kept a good record. Since there's no way to know which detail will turn out to be useful, you have to record all the details.
To do this, without the details overwhelming your account, requires forethought and organization. Think out what you're going to do before you do it, lay out your account with headings and subheadings, set up tables for data and preliminary calculations, use cross-references, and so on. When things don't go as planned, cross out, explain why, and start over.
It's important that your live record and your permanent record be the same. Your account is written directly into the lab book, in ink, once and for all, as you work. Nothing should ever be done on loose sheets and then copied into the notebook. If for some reason you must work without your lab book, do your work on loose sheets and then paste those same sheets into your notebook. Enter all data raw, just as it comes from the measuring instrument, and only then do whatever "processing" of the data (averaging, correcting, etc.) that you need to. Try to form the habit of making and recording rough estimates of experimental uncertainty as you go along no measurement is complete without some assessment of its limitations!
This section of your lab writeup recounts what you did and what happened; not what you think we think should have happened! It is never acceptable for any part of your account to have been clipped out, or copied out, of the experiment writeup.
At the end of the lab period, your instructor will want to look over your data and preliminary calculations, in order to spot any glaring oversights, make suggestions for further analysis, and so on. You have to have an account for him to read not just a list of numbers! He will initial your notebook before you leave.
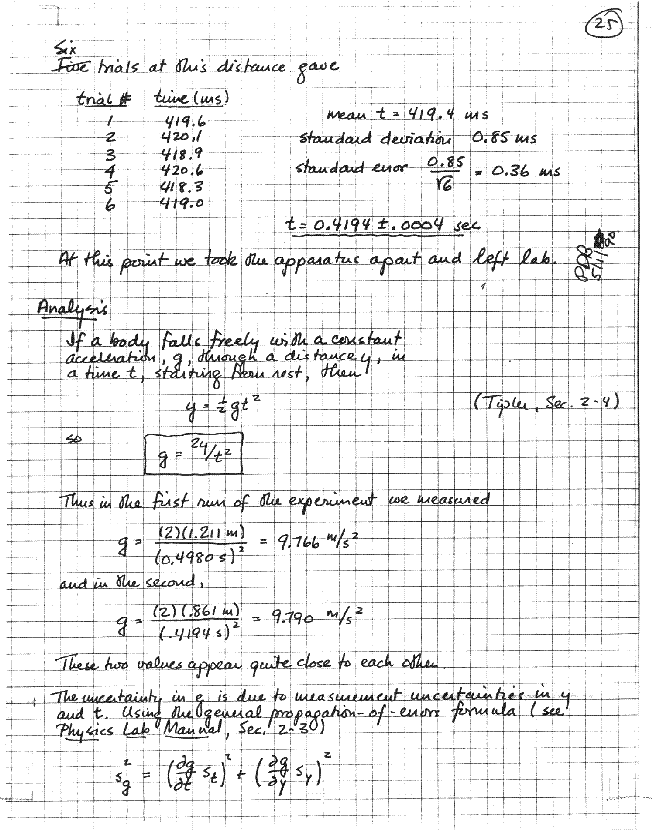
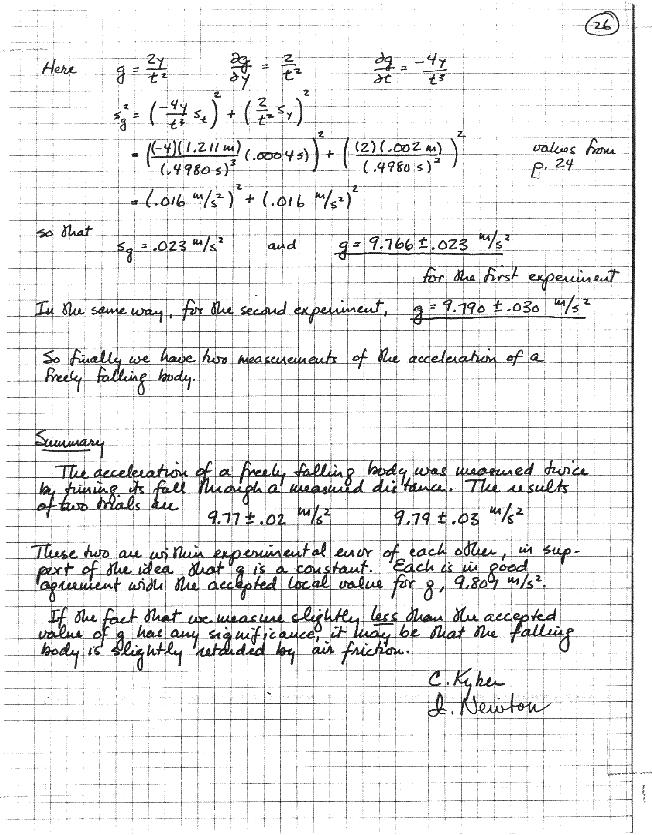
Whatever work you do on your data, after it has been taken, in order to draw conclusions from it. This section will include a description of your methods of analysis, together with any derivations, or reference to outside sources, that this may require; fully worked-out examples of any non-trivial calculations; whatever tabulations, charts, or graphs are needed to express your results, and error analysis. Just like the rest of your writeup, this should be written as the work is done; and, again, thoughtful organization is necessary to keep the main thread of the account from being lost in the details.
Most physics experiments (for that matter, most physics!) deal with the relations between measured quantities. Usually the most expressive way to present such a relationship is graphically. The majority of experiments will require graphs of some sort in the presentation of results. In a later section of this chapter, I'll have more to say about graphs; for now, just notice that the reader looking at your graph should be able to see, without a lot of effort, what quantities are being compared, what relationship you think exists between them, and the degree to which the data you've graphed support that conclusion.
The amount and nature of error analysis that's appropriate varies enormously from one experiment to another. The central purpose, however, is always the same: whatever it is that you've measured, you must understand what you've done well enough so that you can estimate intelligently what uncertainty attaches to your result. This usually involves inferring the magnitude of random errors from your data, resourceful consideration of the origins and magnitudes of possible systematic errors, and whatever transformation and/or combination of errors is called for. Chapter 3 is devoted to these matters.
This section will usually be brief. It should contain clearly and concisely stated whatever conclusions you are led to, from your analysis, about your measurements. In elementary physics labs, there's often a predicted or expected value of whatever you've measured, to compare to. Then your conclusion takes the form of studying your results quantitatively, looking for indications that you botched a calculation or mis-estimated an uncertainty. Did anything happen that you didn't expect, or just don't understand? Can you think of better ways to achieve the object of the experiment, or reduce important sources of error? What we really want to know in this section is - what did you learn from carrying out this exercise?
Make this section concise, specific, pertinent, and quantitative. Above all, avoid buzz-talk like "the discrepancy was only 140%, which is very reasonable for this type of experiment" or "the most important source of error in this experiment was human error." Hand waving can be hazardous to your grade.
End this section with the date on which the writeup was completed, and your signatures. Most instructors will not accept a book that is not signed by both partners.
As an illustration of some of these ideas, study the sample lab writeup that follows. This experiment is briefer and simpler than the ones you'll actually do in PH125, but most of the features it ought to have are here. Discussion, data, and diagram are all interspersed in a single running account of what happened in the lab session; preliminary analysis of the data is carried out on the spot. At first, keeping so detailed an account will seem to take a lot of your lab time; but after a while you'll find that most of your lab writeup everything, probably, except the final analysis and conclusions is already finished when you leave the lab.