
Introductory Laboratory Apparatus
EXPLORING CLOUD FORMATION with a COMPUTER-BASED LABORATORY
Let us begin with a tragic mystery. Sometimes an ominous saucer shaped cloud is seen to descend from a storm cloud as shown below.
Top: Beneath the updraft of a thunderstorm , a "collar cloud" or "wall cloud" forms which appears as an upside-down stump at Tuscaloosa, Alabama. Bottom: A tornado is visible underneath this very large wall cloud, which will kill three people in Linden, Tennessee. Photo courtesy of Curtis Williams at New Albany, Mississippi.
Spotters recognize this feature as a signal that an intense updraft is in this part of the storm, and watch it for the development of a tornado. But how can an upwardly moving air be manifest as a lowering of a cloud?
The process of cloud formation is absent from physics textbooks, yet some of the most fundamental discoveries in physics came about through its study. Although early apparatuses for producing clouds were relatively elaborate, you can instantly demonstrate cloud formation to a sizable audience with an item often found in the garbage. The common polyethylene terephthalate "clear plastic" soda bottle, some water, and a match are all that are needed. The first step is to fill a large two or three-liter soda bottle with enough water to cover the bottom, bringing it to 100% relative humidity. In the atmosphere cloud droplets form around ubiquitous motes suspended in the air, and will need to be replenished for the demonstration to be successful. In order to do this, light the match outside and after compressing the bottle, let it take in air from the environment, or just drop the lit match into the bottle. One can then just blow into the bottle and release the pressure to form a cloud. Also one can seal the bottle with a cap, and strongly grip the bottle for a few seconds. When the pressure is released, a cloud immediately forms inside the bottle, as shown below.

The difference between the clear and cloudy states can be illustrated more vividly be illustrated if the bottle is placed across an overhead projector. If the sealed bottle is compressed again, the cloud will vanish, but is restored upon release. If the cloudy bottle is held next to a Van de Graff electrostatic generator, it will irreversibly vanish. The cloud may be expelled by removing the cap and compressing the bottle, although the cloud fades immediately upon mixing with the environment.
A couple of inexpensive items from an Arbor Scientific kit allows more careful study of the phenomenon of adiabatic heating and cooling, one of the keys to understanding cloud formation. Currently on the market is a fascinating device called a "Fizz Keeper". that keeps soda from going flat. It is a piston that screws on to the neck of a small 355 milliliter or 2 liter soda bottle. Upon pushing the piston several times, the interior is pressurized, which inhibits bubble formation in soda and keeps the carbon dioxide (that makes soda tart and effervescent) dissolved for a longer time. An terrarium thermometer is a strip of liquid crystal digits that respond to temperature within 2o F . When one is placed inside the bottle, it responds very quickly to a change in temperature, with a time constant of a few seconds. Such thermometers have a sticky back and can be kept out of the liquid by sticking it to the upper interior of the bottle. If pumped quickly enough, the interior can be heated to the upper limit of the thermometer of 90o F. When the cap is unscrewed, as the pressure returns to normal, the temperature plunges back to room temperature, or even well below room temperature if the air was allowed to cool to room temperature while pressurized. If we prepare a bottle as before to make a cloud, one realizes that cloud formation corresponds to the sudden lowering of temperature when the air is depressurized.
It would be fascinating to measure the pressure, temperature, and "cloudiness" inside of the bottle for this process. This was done as an introduction to computer-based-laboratory measurements for a physics for education majors course, shown below.

Students in a physics-for-education-majors course learn about computer-based-laboratory measurements with a PASCO 750 interface measuring the pressure, temperature, and optical transmission of a cloud formed inside a soda bottle. Right, Penni Wallace vigorously pumps the bottle after Roger Klee has begun acquiring the data. From right to left, Leslie Beaumont, Tameka Shamery, and Lindsey Couier monitor the data to see if the changes they suggested in the experiment will result in a more successful run.
Pressure, relative optical transmission, and temperature will be monitored inside of a two-liter plastic soda bottle prepared as described above pressurized with a Fizz-Keeper. A PASCO 750 interface will monitor these quantities and analyze the data with the software Data Studio ® by PASCO. . Relative optical transmission was read with a PASCO CI-6504 Light Sensor outside the bottle with a flashlight mounted so that it would shine through the bottle. The sensor consists of an optical cable that is run into a detector box. The light-gathering ability of the cable's head and directional quality was improved by mounting it into the body of a one-time use camera behind the lens. The camera was then mounted opposite to the flashlight. To make measurements inside the bottle, holes were melted in the side by heating a nail and pressing the head against the plastic. This allowed for the spout of an air tube to be connected to the PASCO CI-6532 Absolute Pressure Sensor. For the PASCO CI-6525 High Accuracy temperature probe, the bottle's cap was useful to support it. This was accomplished by drilling a hole through the cap, and mounting it over the hole in the bottle with the thermocouple inserted through the holes. Silicone aquarium sealant was effective in sealing all of the connections, after realizing it was best to put the hoses on the spouts before installing them so as not to extirpate the sealant. One more spout was installed so that the bottle could be slowly depressurized, by slowly unscrewing a Hoffman clamp pinching an exit hose. The experimental setup is pictured below in greater detail.

Pressure, temperature and optical transmission are measured in a soda bottle with a PASCO 750 interface running PASCO Data Studio.
The students made several trials on the system to get a successful run, and prevailed even though the bottle sprang a leak at certain pressure (this difficulty has been eliminated with a more careful job of sealing since). One common misconception about a true Computer-Based Laboratory (CBL) is that one just clicks a button, and the computer will automatically acquire a meaningful experiment. One of the particular educational opportunities of this experiment was the very different time scales of each of the transducers. The light sensor responds so quickly that an ac light source cannot be used. On the other hand, the thermocouple responds so slowly that one must perform the experiment sufficiently slowly that it has time to "catch up" yet quickly enough so that the trial is practically adiabatic. Issues similar to those encountered in this experiment were thoroughly discussed in a recent article in The Physics Teacher by Francis X. Hart in which students investigate the solidification of lard.
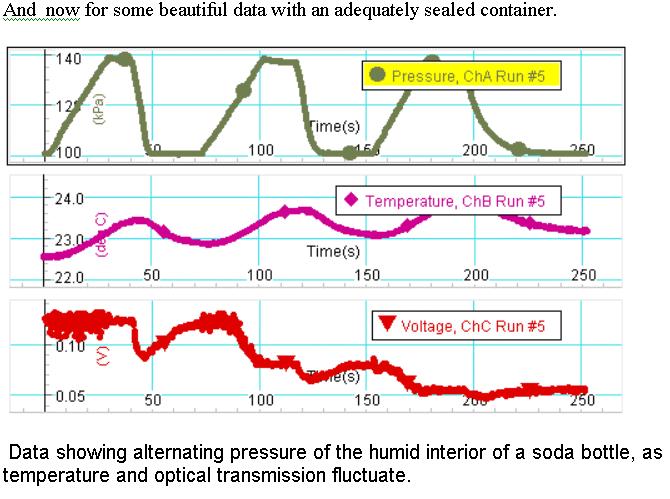
The top chart shows the increase in pressure as the Fizz Keeper pumps the interior to 140 kPA. In the graph beneath it, the temperature rises accordingly by about 1oC. Meanwhile, plenty of light gets through with some fluctuation, probably because of jostling the bottle as it is being pumped. The pressure is lowered by unscrewing a hose clamp, and the temperature drops. The optical transmission drops as a cloud is formed in the bottle. Notice that although the pressure returns to its ambient value, the temperature remains above its ambient value. This illustrates an important concept in weather forecasting, that for the same air mass, it is unlikely that temperature will drop significantly below dew point, because the water droplets releases enthaphy into the air as they form, and is why temperatures vacillate less in more humid regions. The optical transmission increases somewhat, perhaps because droplets begin to aggregate and stick to the container. The bottle is pressurized again, and the opacity (cloudiness) is even greater than before as evident by even less optical transmission. The temperature resides at even higher ambient temperature. As the system is pressurized a third time, the ability to make the cloud disappear is most clearly evident, which upon release of the pressure leaves the lowest optical transmission (thickest cloud) of all.
We can now explain why clouds form, in addition to the answer to our mystery. As air rises the atmospheric pressure is decreased, which cools to dew point, at which the cloud forms with the help of particles in the air to serve as condensation nuclei. The level at which this occurs, the lifted condensation level, corresponds to the base of a cloud. We infer from the presence of an unusually low lifted condensation level that the region is at unusually low pressure, corresponding to upwardly accelerating air.
Acknowledgments
Tremendous thanks are due Craig Bohren of Penn State University, and Charles Knight of the National Center for Atmospheric Research for their tremendous assistance in writing this article. Thanks are also due Gene Byrd and Pieter Vissher and Stan Jones of the University of Alabama for their suggestions toward this article's improvement. I am grateful to University of Alabama's Imaging Services for assistance in photographing the article, and our dedicated library staff in finding some of the references utilized for this article.
References
Apparatus Title: The Gas Menagerie
Abstract
A collection of low cost, easy-to-make apparatuses to illustrate gas principles.
Equipment required to construct apparatus:
Item Source/Store Part Number Cost : Details included with associated article.
Total cost........................................................ : $24.40...................$
Sketch of the apparatus: Pictures included with associated article