Mary V. Frohne
Dept. of Mathematical and Physical Sciences
Benedictine University
5700 College Road
Lisle, IL 60532
(630) 829-6554
Advanced Laboratory and/or Lecture Demonstration Apparatus
and Low-Cost
Apparatus Title: Handy Self-Contained E=hf Demonstrator
Abstract. This apparatus uses a set of different
colored LED's and a strip of phosphorescent tape to demonstrate that photons
of different wavelength also have different energies. Only the blue LED
can excite the phosphorescence. Light of long wavelength quenches the phosphorescence.
Support required for apparatus:
None other than set-up, except that this apparatus needs
to be operated in a darkened room. For the purposes of the apparatus competition,
the apparatus can be operated inside a medium-sized cardboard box with
viewing holes, which I shall send along with the apparatus.
Approximate size: 12 cm x 6 cm x 7.5 cm, not including
a flashlight and a medium size cardboard box with a couple of holes in
it for display (to provide a dark enclosure). With surrounding box to provide
a dark environment, <= 50 cm in any dimension. I am contemplating making
another version of the apparatus, which will be comparable in overall size
to the original but which may have somewhat different linear dimensions.
Does this apparatus require Electrical Power? yes___ no
_X__
Will you be present to set up your apparatus? yes ___
no _X__
When do you plan to set up your apparatus?
Sunday July 22, 9-12 AM_____ 1-5 PM_____
Other support needed for the proper operation of this
apparatus: none
Apparatus description:
Modern physics demonstrations are usually costly and difficult
to set up. This apparatus provides a simple demonstration of the relationship
between photon energy and the frequency, or color, of light. The E=hf demonstrator
consists of a small box with a hinged lid. When the lid is closed, a strip
of glow-in-the-dark (GITD) tape is put into contact with a row of different
colored light-emitting diodes (LEDs). Two demonstrations can be performed
with this apparatus. Both demonstrations are performed in a darkened room
with a small audience.
-
In the first demonstration, the GITD tape has been held in
the dark for a while and is not glowing initially.
-
Close the lid, push the button briefly to turn on the LED's,
open the lid, and one immediately sees that only the section of tape illuminated
by the blue LED is glowing. This is because the light of the other colors
has insufficient energy to excite the phosphorescence.
-
In the second demonstration, the GITD tape is exposed to
white light so that it is glowing uniformly.
-
Turn off the white light, close the lid, push the button
to activate the LEDs for a few seconds, open the lid, and one immediately
sees that the red LED has quenched the glow. This presumably occurs because
the red light is sufficient to excite electrons out of the metastable state
in ZnS and into a state from which they promptly decay into the valence
band. The vast majority of commonly available glow-in-the-dark items rely
on copper-doped zinc sulfide to provide the phosphorescence.
Therefore the key to both demonstrations is a basic understanding
of the phosphorescence mechanism in ZnS. A very simplified explanation
can be given as follows: At least three energy levels are involved. An
electron is excited from the ground state to the top level, and from there
it decays to the middle level. Because transitions from the middle level
to the ground state are forbidden, the electrons get "stuck" in the middle
(metastable) energy level. Eventually they do decay back to the ground
state, giving up light (the phosphorescent glow) in the process. One can
note that the yellow-green color of the phosphorescent glow corresponds
to a longer wavelength than the blue light needed to excite the glow. The
reason why red light quenches the glow is presumably because the red light
is sufficient to excite electrons from the middle metastable state to a
slightly higher state, from which the electrons decay promptly. Although
the previous explanation is sufficient to convey the general idea, it begs
the question of why the electrons get "stuck" in the first place. There
is a vast body of literature on ZnS phosphorescence, and the answer to
this question is complicated because ZnS is a semiconductor . Traditional
explanation of phosphorescence address atoms with metastable states. However,
this explanation is not accurate for ZnS phosphorescence which involves
the movement of both electrons and holes. ZnS is a semiconductor with a
band gap of 3.6 eV between the normally full valence band and the normally
empty conduction band. Doping the ZnS with copper provides additional sites
for electrons to reside at an "energy level" within the band gap. Illuminating
the ZnS with light of sufficient energy excites valence band electrons
to the normally empty conduction band by several routes. The holes in the
valence band are filled with electrons obtained from the copper sites.
Emission from the conduction band to the intermediate-energy copper site
cannot proceed until a hole at a copper site becomes available. Electrons
from the copper site cannot readily drop down into the valence band, which
is normally full. When conduction band electrons finally do combine with
the holes at the copper sites, the ZnS glows with light with a peak wavelength
of 540 nm.
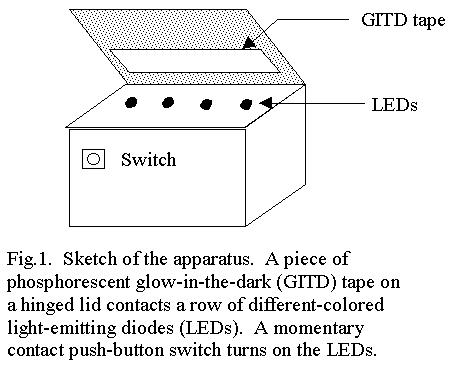 As
seen in Fig. 1, the construction of the apparatus is very simple. The LED's
were all selected to have clear lenses, so that the color of the emitted
light was due entirely to the characteristics of the semiconductors from
which the LEDs were made. The LEDs are arranged in a single row on top
of a small electronics project box. The box is provided with a hinged lid.
A strip of GITD tape is mounted on the underside of the lid so that it
contacts the LEDs when the lid is closed. A resistor chain voltage divider
attached to a 9V transistor radio battery is used to provide the voltage
and current to each LED which is suggested by its manufacturer. A single
momentary contact pushbutton switch completes the electronics.
As
seen in Fig. 1, the construction of the apparatus is very simple. The LED's
were all selected to have clear lenses, so that the color of the emitted
light was due entirely to the characteristics of the semiconductors from
which the LEDs were made. The LEDs are arranged in a single row on top
of a small electronics project box. The box is provided with a hinged lid.
A strip of GITD tape is mounted on the underside of the lid so that it
contacts the LEDs when the lid is closed. A resistor chain voltage divider
attached to a 9V transistor radio battery is used to provide the voltage
and current to each LED which is suggested by its manufacturer. A single
momentary contact pushbutton switch completes the electronics.
The inside of the lid is also fitted with a chart showing
the operating voltages, nominal wavelengths, and nominal luminosities of
the LEDs. These data are available on the packages in which the LEDs were
purchased. The students can calculate the photon energies from these data,
and discuss the lack of effectiveness of luminosity (the red is brightest,
the blue dimmest) on exciting the phosphorescence, as well as the reasons
why the blue LED requires a higher operating voltage than the red.

Fig. 2: This is a photo of the apparatus with its lid
closed. The switch is mounted on top of the box in this version.

Fig. 3: This is a photo of the box with its lid open and
its LEDs activated. The chart shows the peak wavelength, nominal luminosity,
and operating voltage of each LED. The data were obtained from the manufacturer's
LED packaging.
Other uses for this apparatus include applying the lights
to other light-sensitive devices, such as solar cells, photocells, photoresistors,
etc. to test for wavelength dependences in their functioning.
Reference. 1. Lisensky, G. C., Patel, M.D., Reich, M.,
J. Chem. Ed. 73, 1048 (Nov. 1996).
 As
seen in Fig. 1, the construction of the apparatus is very simple. The LED's
were all selected to have clear lenses, so that the color of the emitted
light was due entirely to the characteristics of the semiconductors from
which the LEDs were made. The LEDs are arranged in a single row on top
of a small electronics project box. The box is provided with a hinged lid.
A strip of GITD tape is mounted on the underside of the lid so that it
contacts the LEDs when the lid is closed. A resistor chain voltage divider
attached to a 9V transistor radio battery is used to provide the voltage
and current to each LED which is suggested by its manufacturer. A single
momentary contact pushbutton switch completes the electronics.
As
seen in Fig. 1, the construction of the apparatus is very simple. The LED's
were all selected to have clear lenses, so that the color of the emitted
light was due entirely to the characteristics of the semiconductors from
which the LEDs were made. The LEDs are arranged in a single row on top
of a small electronics project box. The box is provided with a hinged lid.
A strip of GITD tape is mounted on the underside of the lid so that it
contacts the LEDs when the lid is closed. A resistor chain voltage divider
attached to a 9V transistor radio battery is used to provide the voltage
and current to each LED which is suggested by its manufacturer. A single
momentary contact pushbutton switch completes the electronics.

